Page Contents
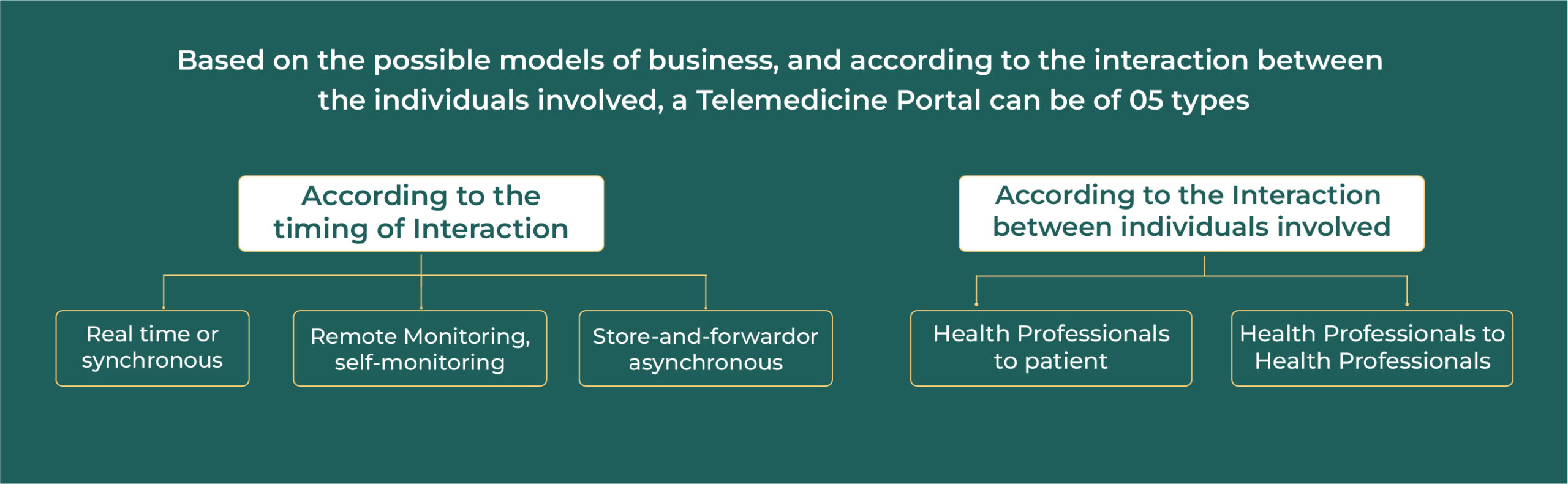
Telemedicine is changing the way healthcare services are delivered. As more and more patients opt for virtual healthcare, it’s crucial for med-tech platforms to comply with telemedicine requirements.
The Notification of the Telemedicine Practice Guidelines (“Telemedicine Guidelines”/ “Guidelines”) as a part of Appendix 5 of the Indian Medical Council (Professional Conduct, Etiquette & Ethics) Regulations, 2002 (“MCI Code”), has made: (a) the practice of the medical profession; and (b) provision of medical care over technology platforms, legal and regulated. These Guidelines impact a cross-section of stakeholders, such as medical professionals (“MP”), registered medical practitioners (“RMPs”), patients, caregivers and med-tech platforms.
While med-tech platforms are primarily responsible for ensuring that the MPs providing services comply with the ethical and legal aspects of telemedicine, they must also abide by the relevant laws and regulations. The Guidelines are for guidance purposes, laying out the primary principles, i.e. the contours within which telemedicine practice in India is to be followed. However, the Guidelines need to be read in conjunction with other applicable laws.
The laws that med-tech offering telemedicine services in India must comply with include: (a) the Indian Medical Council Act, 1956 (MCI Act) and the MCI Code; the Drugs and Cosmetics Act, 1945 and Rules made thereunder (D&C Act); the Telecom Commercial Communication Customer Preference Regulations, 2018 (TCCP Regulations); the Consumer Protection Act, 2019 (CPA); and the Foreign Exchange Management Act, 1999 (FEMA).
In conclusion, while the Guidelines are crucial, the med-tech platforms offering telemedicine services must comply with the necessary ethical and legal aspects of telemedicine in order to avoid penalties and potential liabilities. Before implementing tech-based solutions for telemedicine, businesses should evaluate the mandatory requirements and ensure compliance with relevant laws and regulations, in order to reduce potential liabilities.
FAQ’s
Q: How to start a telemedicine service in India?
A: Before starting telemedicine services in India, med-tech platforms must comply with telemedicine requirements laid out by the Ministry of Health and Family Welfare and NITI Aayog. They must evaluate the nature of services and ensure compliance with the relevant laws and regulations, such as the Indian Medical Council Act, 1956 and the Indian Medical Council (Professional Conduct, Etiquette & Ethics) Regulations, 2002 the Drugs and Cosmetics Act, 1945 and Rules made thereunder; the Telecom Commercial Communication Customer Preference Regulations, 2018; the Consumer Protection Act, 2019; and the Foreign Exchange Management Act, 1999.
Q: What are the requirements of telemedicine standards?
A: The requirements of telemedicine standards in India contain a set of Telemedicine Practice Guidelines (“Guidelines”) as part of Appendix 5 of the Indian Medical Council (Professional Conduct, Etiquette & Ethics) Regulations, 2002, which outlines the legal and regulatory aspects with respect to the practice of medical professionals through med-tech platforms, for medical care and consultations. These guidelines provide legal and ethical frameworks and impact various stakeholders like medical professionals, registered medical practitioners, patients, caregivers, and med-tech platforms.
Q: What are the protocols used in telemedicine services?
A: Telemedicine services transmit medical information from the patient to the doctor via telecommunication technology as per the applicable laws. The protocol used in telemedicine services depends on the type of service provided, including audio-only consultation, video consultation, or text-based services. These protocols combine the use of equipment such as smartphones, tablets, laptops, and medical devices to assist edical professionals in providing the necessary healthcare services.
Q: Are telemedicine services legal in India?
A: Yes, telemedicine services are legal in India provided that the businesses offering med-tech platforms comply with the Telemedicine Practice Guidelines (“Guidelines”) as a part of Appendix 5 of the Indian Medical Council (Professional Conduct, Etiquette & Ethics) Regulations, 2002, in addition to other relevant applicable laws and regulations. Med-tech platforms offering telemedicine services must evaluate the nature of services and comply with necessary legal and ethical aspects of telemedicine, in order to reduce potential liabilities and ensure better and qualitative healthcare.
Looking for expert contract advice? Call us at +91 9930156000 today
Disclaimer – The content of this document is for information purpose only and does not constitute advice or a legal opinion. It is based upon relevant law and/or facts available at that point of time and prepared with due accuracy & reliability. Readers are requested to check and refer to relevant provisions of statute, latest judicial pronouncements, circulars, clarifications etc. before acting on the basis of this write up. The possibility of other views on the subject matter cannot be ruled out. By the use of the said information, you agree that the Treelife is not responsible or liable in any manner for the authenticity, accuracy, completeness, errors or any kind of omissions in this piece of information for any action taken thereof.